Our Research Projects

Spatial Biomarkers of early Alzheimer’s disease (SABRE)
This study, funded by the National Institute for Health Research (NIHR), aims to develop spatial tests for diagnosis of early AD in routine clinical practice. The tests of spatial navigation and memory used in our research studies, developed by out ICN colleagues Neil Burgees and Andrea Castegnaro, will be adapted to create short, user-friendly versions suitable for use in GP practices and memory clinics. To achieve this we will develop an Augmented Reality (AR) version of a Virtual Reality (VR) path integration task and will be working with design engineers at The University of Cambridge and public-patient initiatives overseen by The University of Newcastle. To ensure inclusivity and usability in diverse populations, the new tests will be trialled in patients recruited from various memory clinics across the UK serving different ethnic minorities and demographics.
Cross-species studies of EC-hippocampal function in early AD
Funded by a UK Dementia Research Institute (UKDRI) Grand Challenge prize, this work bridges mouse model and human AD research. There are two parallel aims: first, to establish the cellular basis for the path integration impairment observed in early AD and second, to determine whether altered path integration is the initial cognitive deficit at AD onset. The study co-investigators are UCL colleagues Professor Karen Duff (UKDRI and study lead), Nobel Laureate Professor John O’Keefe (Sainsbury Wellcome Centre), Dr Julija Krupic (UKDRI) and Dr David Aguillon (University of Antioquia, Colombia).
VR path integration is tested in people at risk of sporadic and familial AD. UK testing is combined with 7T functional MRI measures of EC function while for familial AD work this study benefits from access to the world’s largest cohort of autosomal dominant AD in Colombia.
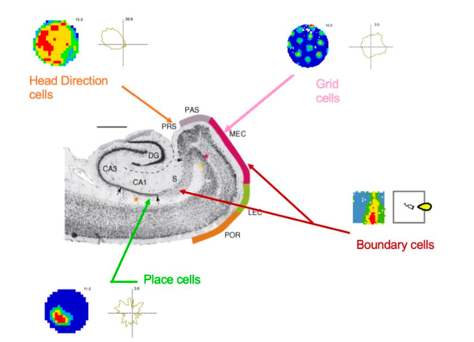

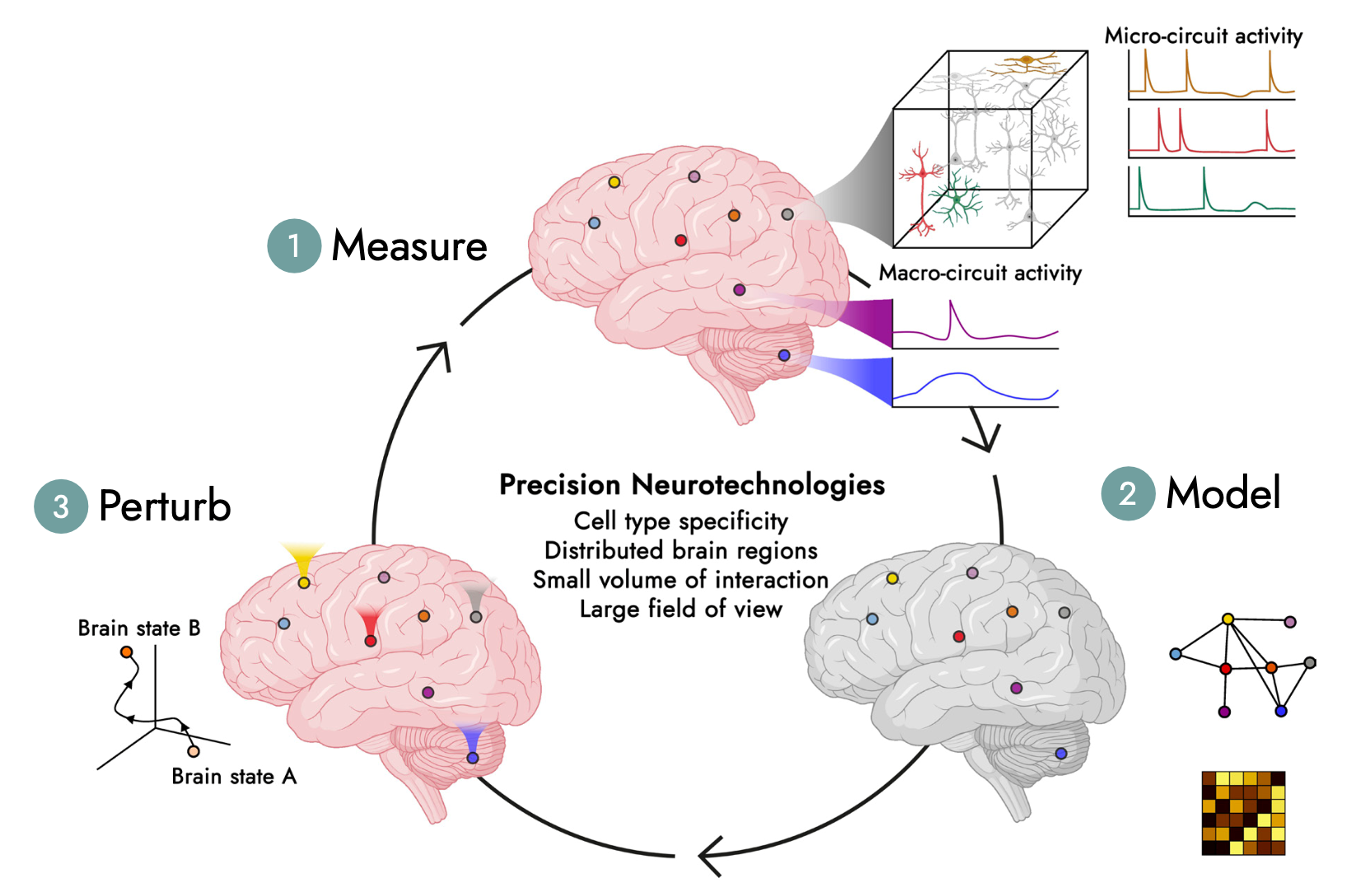
Their clinical implementation will be guided by our ethics-centred systems framework.
An ethics-based systems framework for future clinical use of precision neurotechnology
Next generation precision neurotechnology may transform the management of neurological diseases in their potential to alter brain circuit function and ameliorate the effect of disease. Funded by ARIA (Advanced Research + Invention Agency) we are tasked with delivering an ethics-centred framework, based on systems engineering principles, within with neurotechnologies emerging from ARIA’s will be implemented into clinical practice. Such a framework is crucial for addressing potential ethical considerations associated with use of next generation technologies, minimising risk and maximising benefit to patients and clinicians. For this work the Chan lab partners with Professor John Clarkson (Department of Engineering, University of Cambridge) and Dr Richard Milne (Kavli Centre for Ethics, Science and the Public, University of Cambridge).
AHEAD (At Home EEG screening for early detection of Alzheimer’s Disease)
Disruption of sleep occurs early in AD and its detection may improve not just diagnostic sensitivity but also specificity in distinguishing AD from other dementia causing diseases in which sleep is affected differently. The emergence of ear-EEG technologies, capable of remote capture of sleep data, could transform measurement of sleep changes in AD and other conditions in permitting at-home recording without the inconvenience and limited availability of current sleep labs. The AHEAD study, funded in part by a grant from Innovation Fund Denmark to our Danish collaborators Professor Preben Kidmose (University of Aarhus) and Dr Martin Hemmsen (T&W Engineering), will test the ability of ear-EEG to classify early AD.
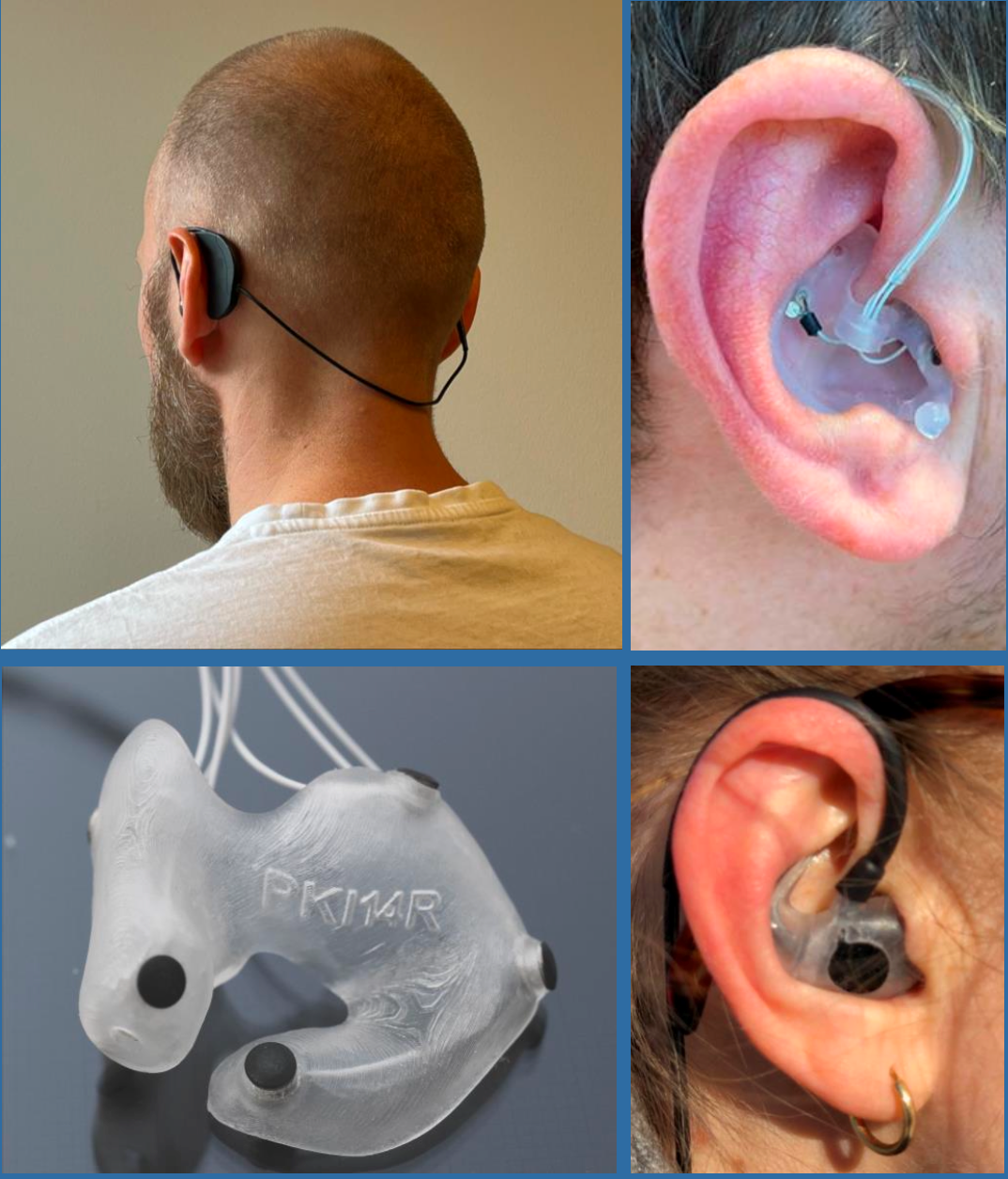
(Top right and bottom right). The ear-EEG devices in situ.